Atomic structure diagram
The atomic structure is drawn with the nucleus in the center, and electrons arranged outside in circles called energy levels or shells. The electrons in an atom occupy the innermost available shells, i.e. the lowest available energy levels.
The innermost shell, or the first shell, can hold only 2 electrons.
The second and third shells can hold up to 8 electrons each.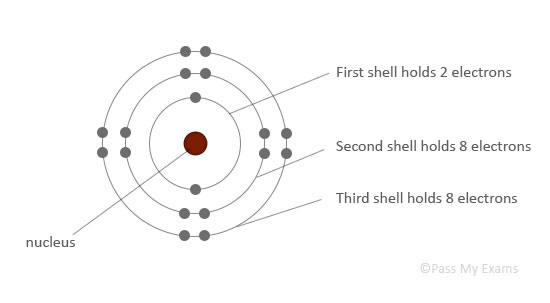
First shell (closest to nucleus)
2 electrons
Second shell:
8 electrons
Third shell:
8 electrons
Elements: Atomic Structure Diagram
Page: 1
Posts 1 to 1 of 1
Share12019-01-26 17:04:01
Page: 1