Atomic Number
Each atom of a specific element contains the same number of protons. For example, all the atoms of carbon contain 6 protons in their nucleus.
The number of protons in an atom of an element is called Atomic number.
Each element has a unique atomic number, which is also used to arrange the elements in the Periodic Table.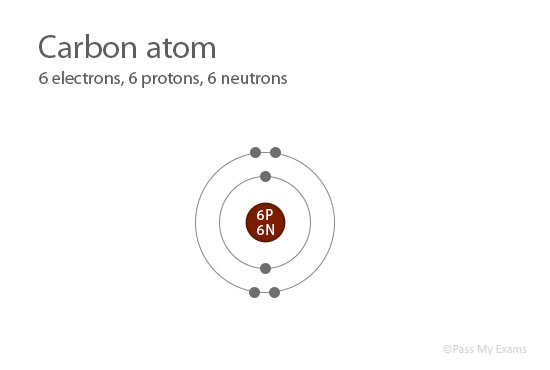
Atomic Number = Number of protons
= 6
Carbon has 6 protons; therefore the atomic number of carbon is 6.